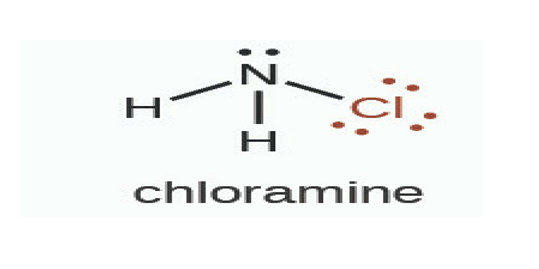
MONOCHLORAMINE
Chloramines are a group of chemical compounds that contain chlorine and ammonia. The particular type of chloramine used in drinking water disinfectant is called monochloramine which is mixed into water at levels that kill germs but are still safe to drink.
MOLECULAR STRUCTURE
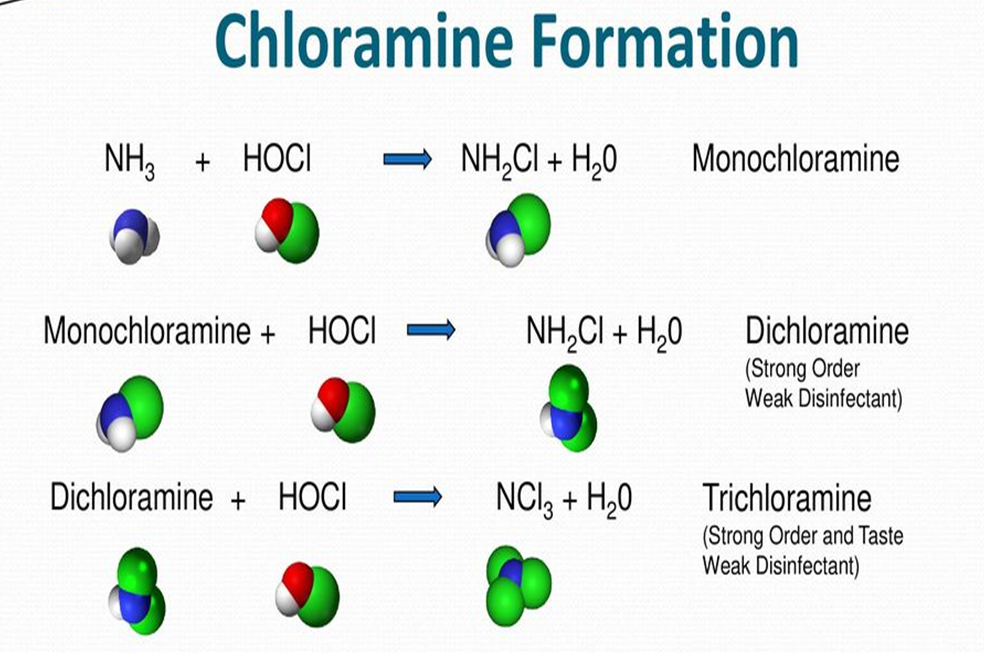
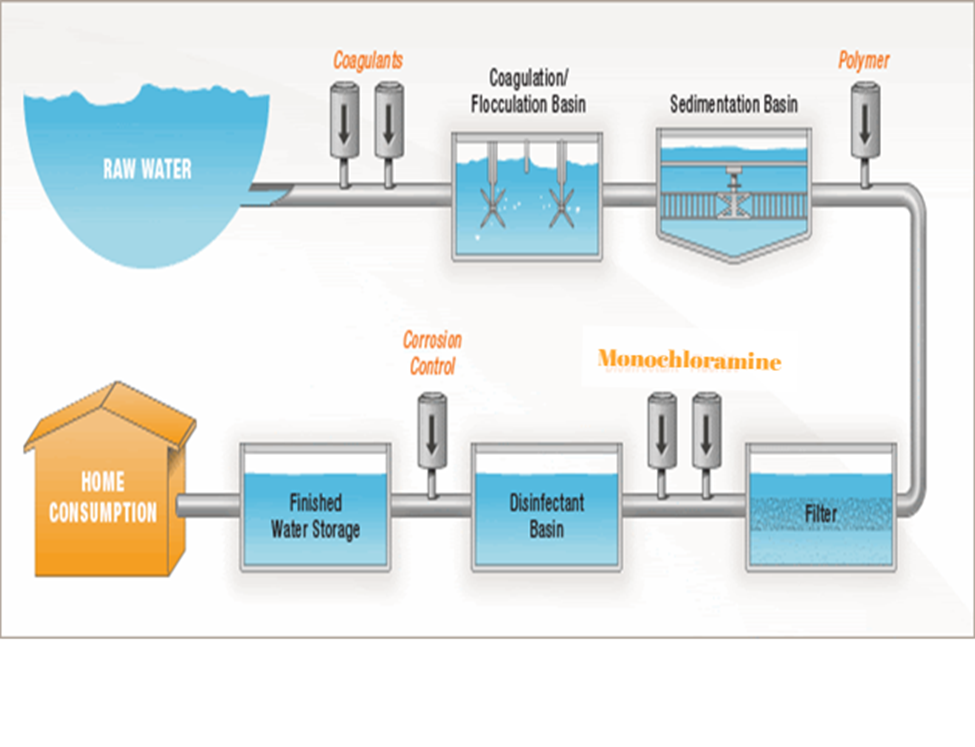
CHLORINE/CHLORAMINE TREATMENT – WATER TREATMENT SYSTEM
pH and Monochloramine
If the pH of water is above 7(neutral) then you naturally have monochloramine- the “chloramine” as used by water industry.
If pH is lower, get bi-chloramine and tri-chloramine
Drinking water is pH adjusted.
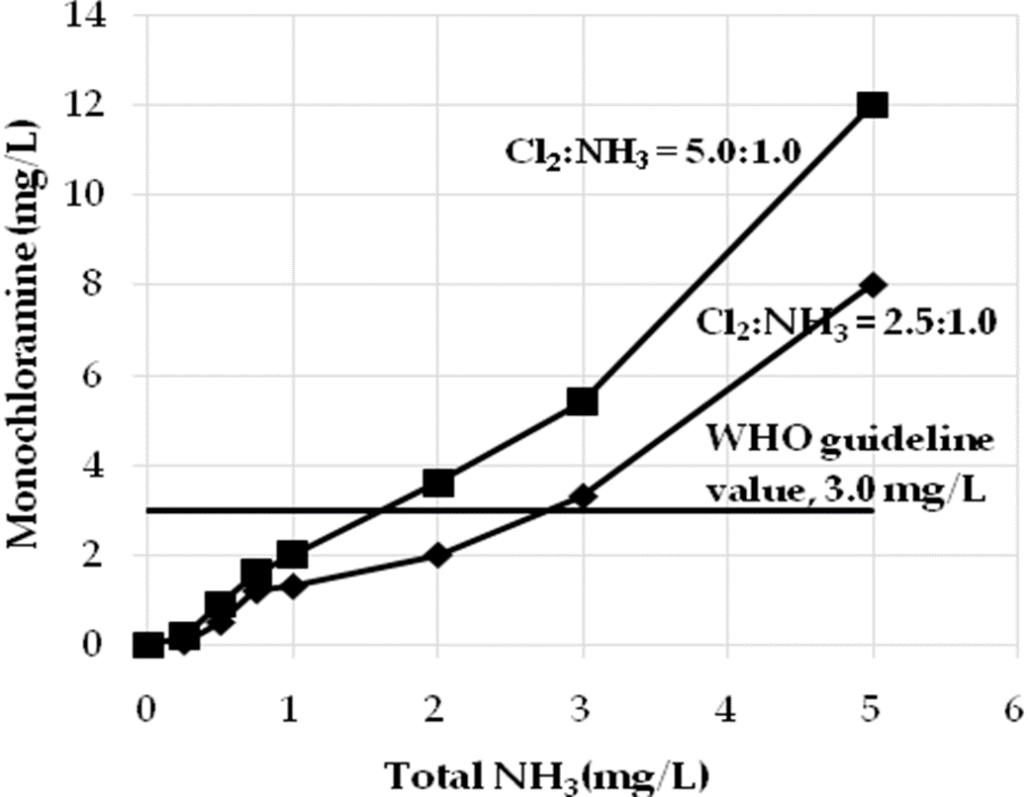
Fig: Concentration of monochloramine produced during chlorination of water.
SAFE LEVELS OF CHLORAMINE IN WATER
Chloramine levels upto 4 milligrams per litre(mg/L) or 4 parts per million(ppm) are considered safe in drinking water.
At these levels, harmful health effects are unlikely to occur.

CHLORINE VS MONOCHLORAMINE
| S.NO | CHLORINE | MONOCHLORAMINE |
| 1 | Chlorine is a gaseous compound having a yellow green color and it is a toxic gas. | Chloramine is a gaseous compound and it is comparatively less toxic gas. |
| 2 | Chlorine dissipates and evaporates into the air quickly. Chlorine will usually evaporate after sitting for 24 hours. | Chloramine is more stable and will last longer in the water system. |
| 3 | Chlorine inactivated, no power to kill microbes at end of water delivery pipe. | Chloramine still present, killing microbes at end of water delivery pipe. |

