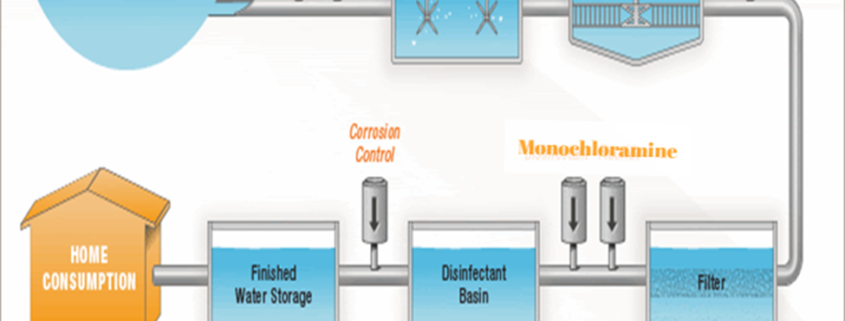
MONOCHLORAMINE-INTRODUCTION & APPLICATION
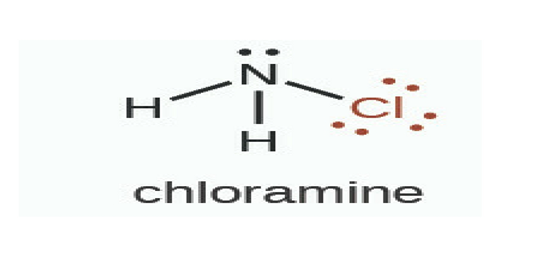
Chloramines are a group of chemical compounds that contain chlorine and ammonia.
The particular type of chloramine used in drinking water disinfection is called monochloramine which is mixed into water at levels that kill germs but are still safe to drink.
pH and Monochloramine
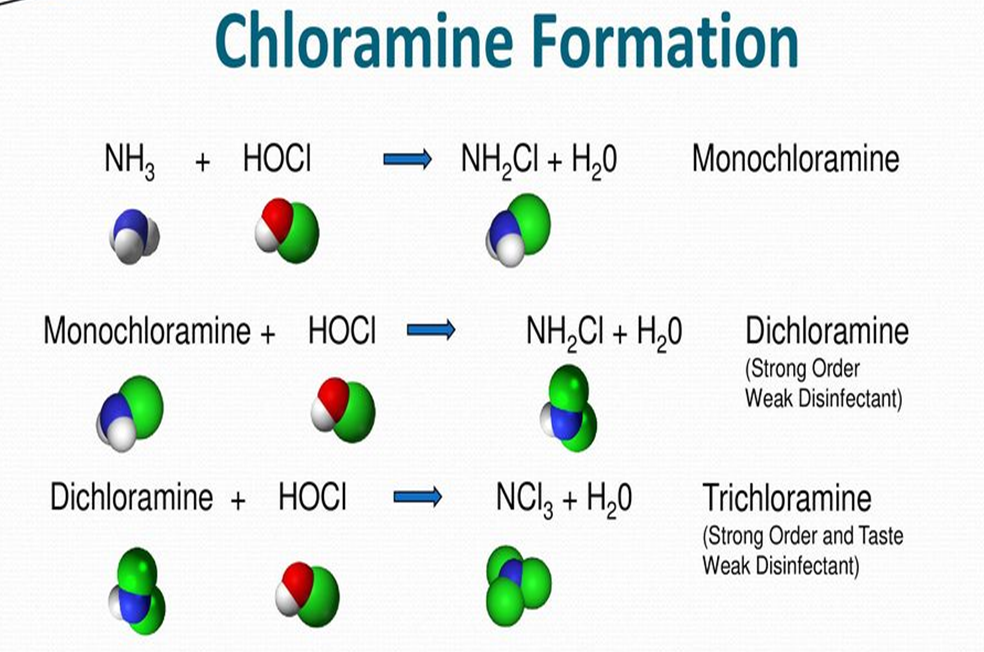
- If the pH of water is above 7 (neutral) then you naturally have monochloramine- the “chloramine” as used by water industry.
- If pH is lower, get bi-chloramine and tri-chloramine.
- Drinking water is pH adjusted.
Safe levels of Chloramine in water
- Chloramine levels up to 4 milligrams per liter (mg/L) or 4 parts per million (ppm) are considered safe in drinking water.
- At these levels, harmful health effects are unlikely to occur.
CHLORINE VS MONOCHLORAMINE
| S.NO | CHLORINE | CHLORAMINE |
| 1 | Chlorine is a gaseous compound having a yellow green color and it is a toxic gas. | Chloramine is a gaseous compound and it is comparatively less toxic gas. |
| 2 | Chlorine dissipates and evaporates into the air quickly. Chlorine will usually evaporate after sitting for 24 hours. | Chloramine is more stable and will last longer in the water system. |
| 3 | Chlorine inactivated, no power to kill microbes at end of water delivery pipe. | Chloramine still present, killing microbes at end of water delivery pipe. |
APPLICATIONS
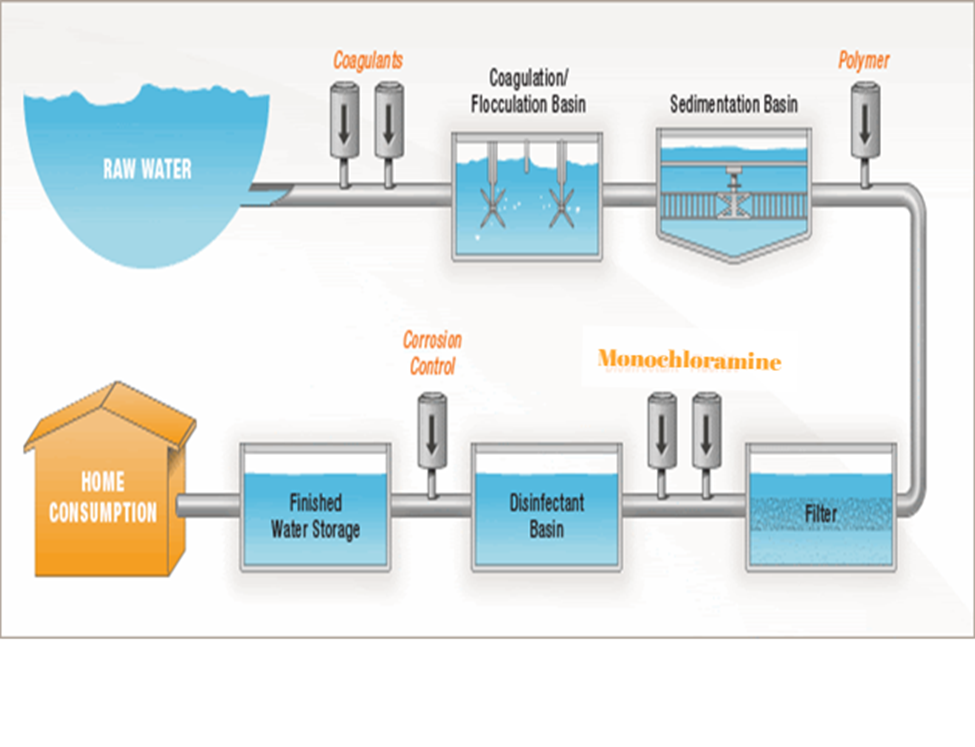
DRINKING WATER
- Monochloramine is favored because it is less reactive and remains in solution longer, providing added protection from contamination.
- It is safe for dialysis patients to drink, cook with and bathe in monochloraminated water because the digestive process neutralizes monochloramine before it enters the bloodstream.
- Monochloramine is emerging as an integral part of controlling the growth of Legionella bacteria
Chloramine has been used as a drinking water disinfectant in the United States in places like Cleveland, Ohio, Springfield, Illinois, and Lansing, Michigan since 1929. In 1998, an EPA survey estimated 68 million Americans were drinking water disinfected with chloramine. Several major U.S. cities such as Philadelphia, San Francisco, Tampa Bay, and Washington, D.C. use chloramine to disinfect drinking water. Chloramine is recognized as a safe disinfectant and a good alternative to chlorine.
More Information
EPA: Drinking Water Regulationsexternal icon
EPA: Drinking Water Treatability Databaseexternal icon
EPA. Chloramines in Drinking Waterexternal icon
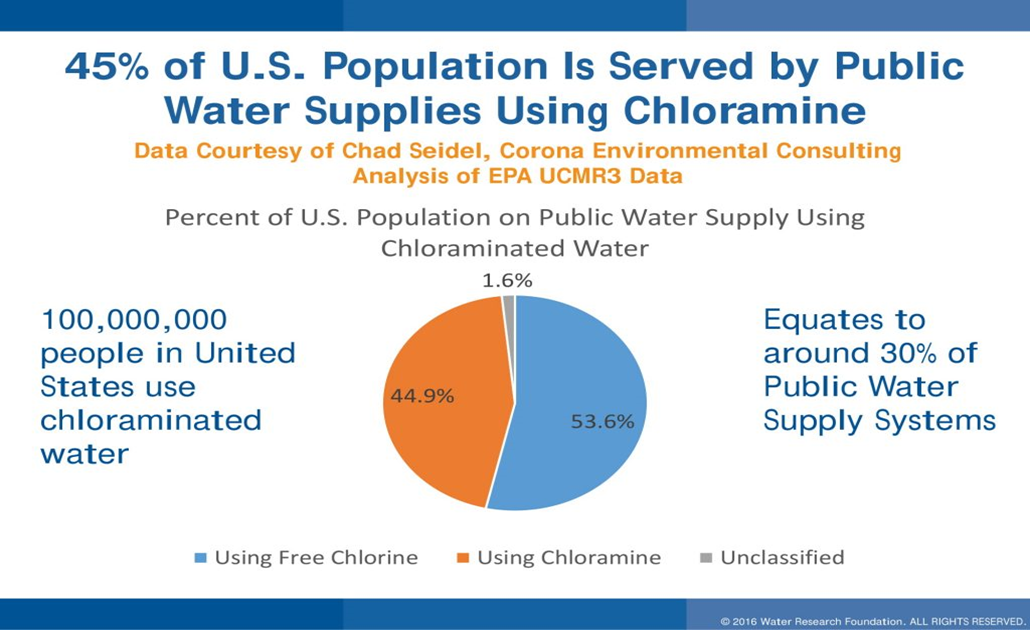
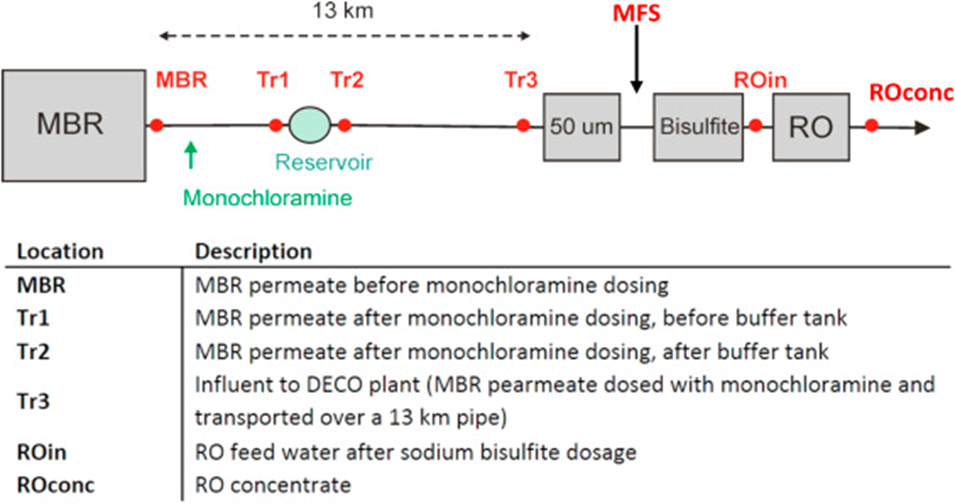
Monochloramine for wastewater reuse
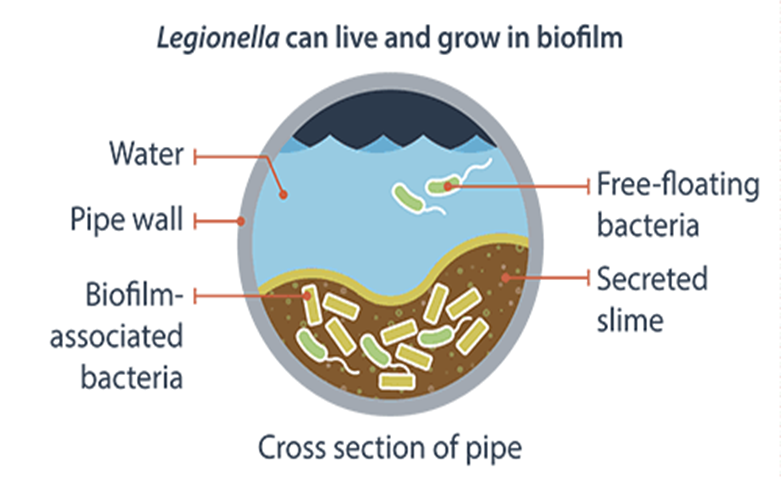
Legionella growth and spread
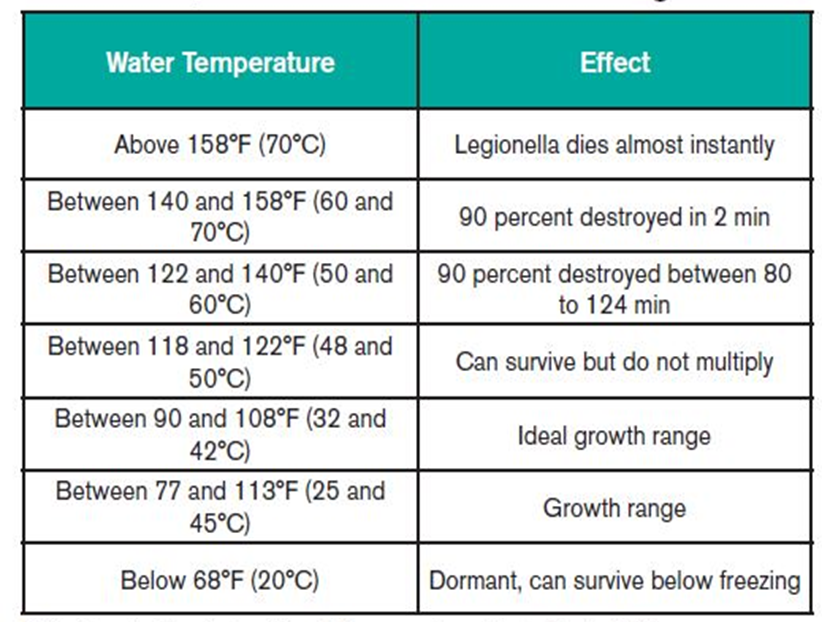
- Legionella bacteria is commonly found in water. The bacteria multiply where temperatures are between 20-45°C and nutrients are available. The bacteria are dormant below 20°C and do not survive above 60°C.
- Legionella reside in water distribution pipes and can live in premise water tanks where temperatures are between 20ºC and 50ºC
Temperature affects the survival of Legionella
Monochloramine Use for Prevention of Legionella
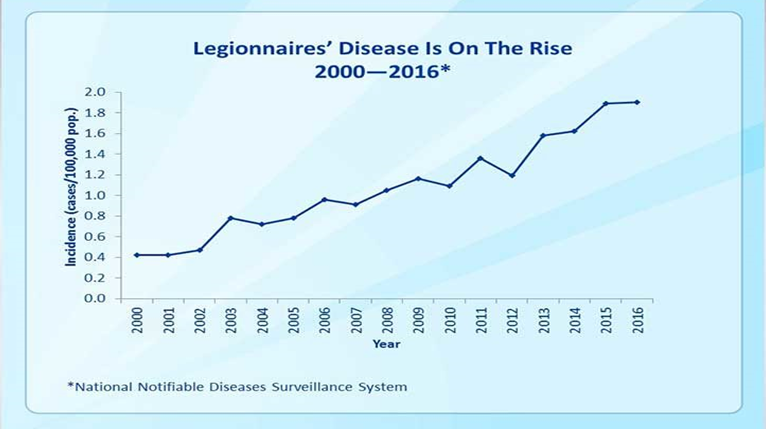
- Monochloramine disinfection of municipal water supplies is associated with decreased risk for Legionnaires’ disease.
- Increasing use of monochloramine in water supplies throughout the United States may reduce Legionella transmission and incidence of Legionnaires’ disease.
- Controlling water temperatures is one of the most effective ways of preventing the growth and spread of Legionella bacteria.
- The ideal temperature range for the growth of the bacteria is between 20 and 45 degrees Celsius. Outlet temperatures for cold water should therefore be kept below 20 degrees Celsius, and hot water outlets above 50 degrees Celsius to control the bacteria.
EU
The European drinking water guideline does not contain standards for chloramines. When chloramines are used, few disinfection byproducts, such as trihalomethanes, are formed. However, other disinfection byproducts can form. Examples are toxic halonitrils (cyano chloride), halonitromethanes (chloropicrin) and other nitrogen-rich compounds. Some of these compounds can endanger human health. When the European Drinking Water Directive is revised, standards for these compounds will be added.
USA
According to American guidelines by EPA, drinking water that is treated with chloramines can contain a maximum amount of 4 mg/L Cl2. (National Primary Drinking Water Regulations EPA, 2002)
WHO
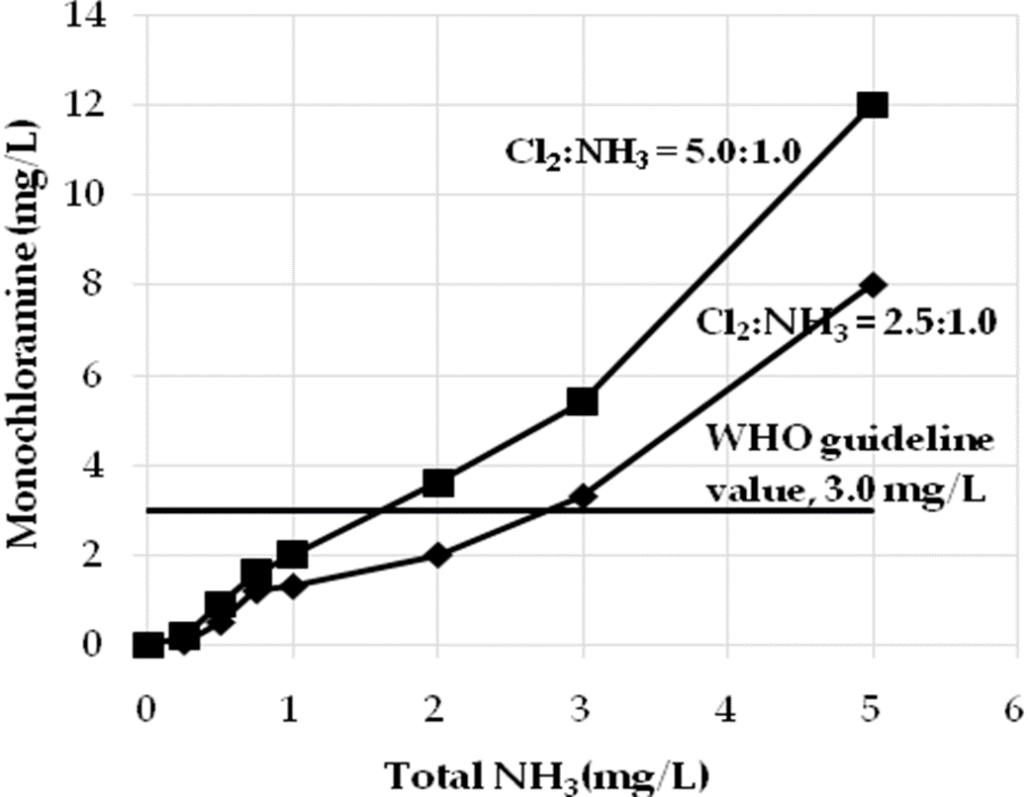
The WHO (World Health Organization) only dictates a standard for monochloramine as a disinfectant. The standard is 3 mg/L. For di- and trichloramine there are no standards, because the available information is not satisfactory for the establishment of a health guideline. (WHO, Guidelines for drinking-water quality – 3rd edition. Chemical aspects).



Leave a Reply
Want to join the discussion?Feel free to contribute!